So interesting story, just was about to write my blog and a minor earthquake just happened. Anyway the biochemistry must be go on!
So today we will be doing denaturation. This is going to be very short because the explanations are very simple and I am sure you going to catch on very quickly. The factors which affect the rate at which an enzymes catalyze reactions are substrate concentration, enzyme concentration, temperature and pH.
As substrate concentration increases the initial rate of the reaction increase. This is accounted for by the more substrate which are present the more collision will occur between the substrate and enzyme molecules. This substrate concentration increases until there is a maximum saturation point. Where all the enzyme which are present are occupied by the enzyme. This is called the maximum velocity. The enzyme concentration, temperature and pH are kept constant.
As enzyme concentration increases the rate of reaction also increases. In this case the substance concentration, temperature and pH is kept constant. As the enzyme concentration increases the amount of active sites available for the reaction also increases.However this happens to a maximum point. In this case as the concentration of the enzyme increase however the substance concentration remains the same, so not all the active sites are used up. The rate will remain constant at this point.
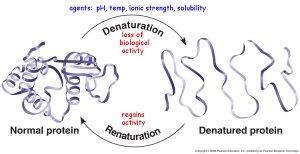
As temperature increases the energy of the system also increases. This leads to an increase in the energy of the molecules which cause more collision between the enzyme molecules and the substrate molecules. This also gives the substrate more energy to overcome the activation energy. However as temperature increases over the optimum temperature, the rate reaction decreases significantly. This is as the enzymes are denatured. High temperatures break the hydrogen bonds in the enzyme structure.
The effect of pH on the enzyme is targeted to the ionization if the active site. In acidic conditions the COO- group can be protonated and in a basic solution the NH4+ can be deprotonated. This will interrupt the ionic interactions between the side chains which increases folding.
So that’s it folks. Have a good day!
Reference:
PatriotAPBio2010 – Unit01-Biochemistry
Patriotapbio2010.wikispaces.com. “PatriotAPBio2010 – Unit01-Biochemistry.” 2013. http://patriotapbio2010.wikispaces.com/Unit01-Biochemistry (accessed 9 Mar 2013).